View Procedure
| Procedure Name | Habit Forming Drugs/Psychotropic – Import Permit | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
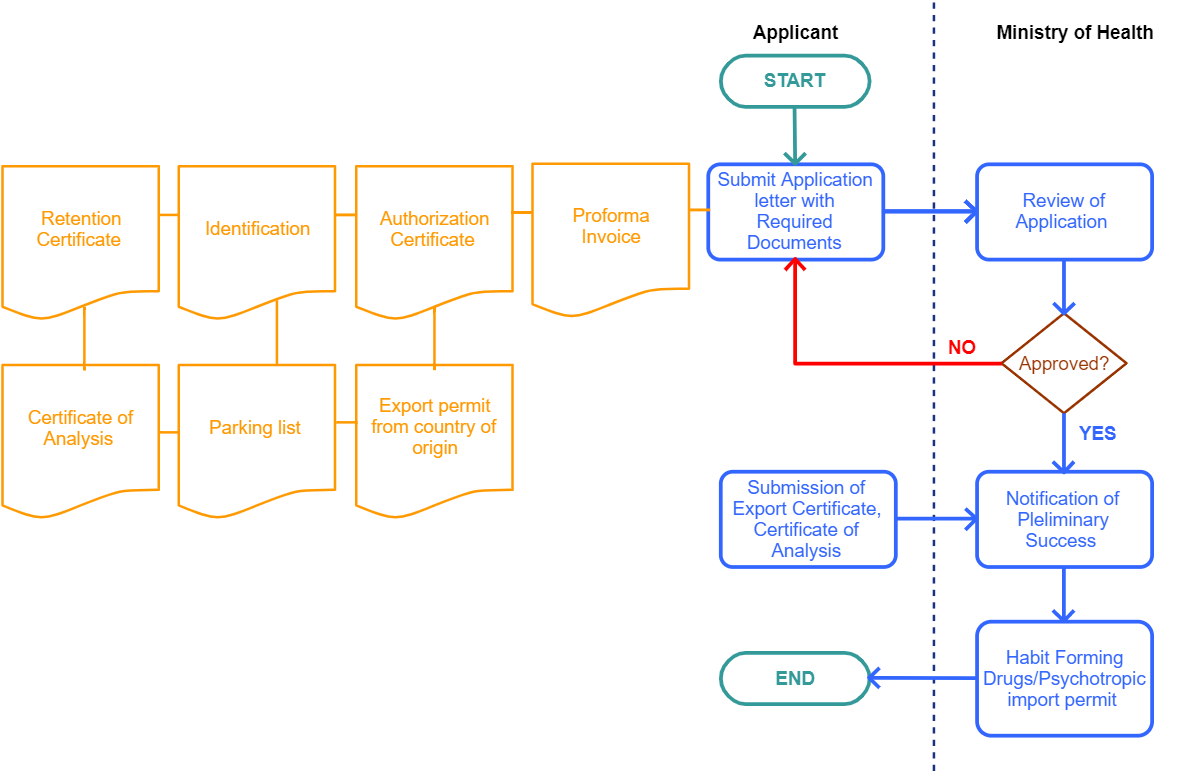
Required Documents
Process Steps
| |||||||||||||||||||||||||||||||||||||||||||
| Category | Procedures |
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Export Permit for Narcotic Drugs and/or Psychotropic Substances | Export Permit for Narcotic Drugs and/or Psychotropic Substances | 22-05-2014 | 22-05-2014 |
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Habit forming drugs/psychotropic import/export permit | Permit Requirement | Habit forming drugs/psychotropic import/export permit | N/A | The Medical, Dental and Pharmacy Order 1970 | 01-01-9999 | Good |